Charting the Cellular Terrain: Spatial Biology's Promise
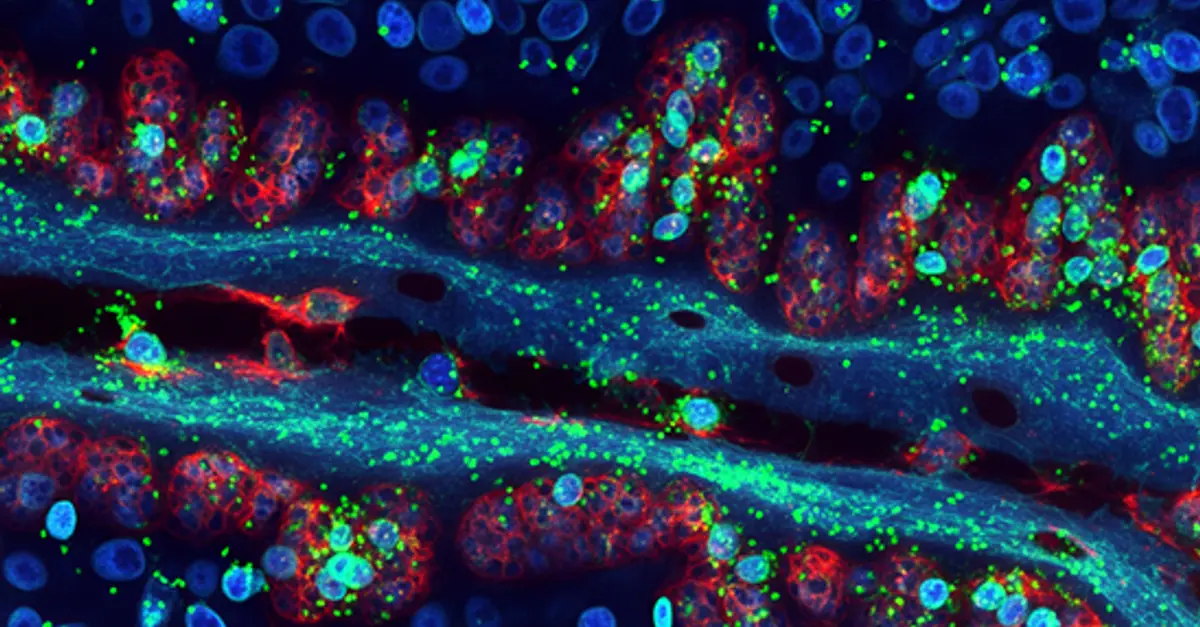
Spatial biology investigates how cells organize within tissues, considering their arrangement, type, and molecular state. Cells within tissues receive internal and external signals that guide their development and behavior, influencing processes like cell division, growth, and specialization. By mapping the location and activity of molecules within tissues, spatial biology provides unparalleled insights into the spatial organization of life. This transformative field is revolutionizing our understanding of fundamental biology and driving the development of novel therapies.
Recent breakthroughs in nuclei isolation and RNA handling from FFPE (Formalin-Fixed Paraffin-Embedded) tissue have unlocked new possibilities for spatial analysis of these valuable samples. Together with the surge in high-throughput omics technologies, scientists can now systematically explore to navigate the molecular layers, gaining insights into the genetic blueprint (genome), gene expression profiles (transcriptome), protein composition (proteome), and metabolic state (metabolome) of cells within their spatial context.
Early approaches, such as in situ sequencing (ISS) with padlock probes or Fluorescent in situ RNA sequencing (FISSEQ), allowed for the targeted or whole-transcriptome profiling of RNA molecules within tissue sections. However, these techniques were limited in resolution and throughput. Spatial transcriptomics, pioneered in 2016, introduced a microarray-based method to capture spatial gene expression information, at a resolution that evolved from 100-μm to 2μm achieving subcellular precision. A powerful technique for imaging RNA molecules in single cells have emerged called MERFISH (multiplexed error-robust fluorescence in situ hybridization), that sequentially labels RNA transcripts with fluorescent probes and image them using via single molecule fluorescence in situ hybridization (smFISH). This spatially resolved, single-cell gene expression profiling could be multiplexed to hundreds and thousands of RNA species by using combinatorial labeling and sequential imaging at single cell resolution.
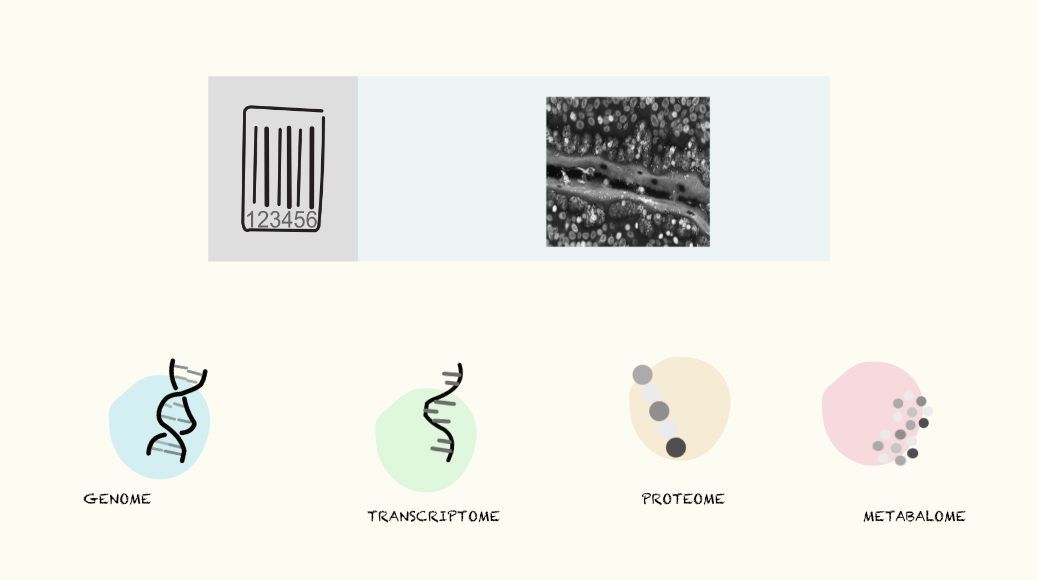

A novel approach developed by scientists at Yale University called 'Deterministic Barcoding' (DBiT-seq) leverages microfluidics to deliver DNA barcodes into tissue sections in a spatially resolved manner, enabling the co-mapping of multiple omics layers, including transcriptomics, proteomics, and epigenomics.
While spatial transcriptomics generally offers higher multiplexing and throughput, spatial proteomics advancements have been historically limited, primarily because proteins are highly variable in shapes and sizes, have complex secondary & tertiary structures and moreover cannot be amplified like DNA. However, recent breakthroughs in antibody-based imaging technologies, including IMC-Cytof (Imaging Mass Cytometry) and MIBI-TOF (Multiplexed ion beam imaging by time-of-flight) have significantly enhanced the ability to simultaneously detect multiple proteins in spatial context. Further, scientists have adapted highly sensitive LC-MS (liquid chromatography mass spectrometry) -based proteomics to spatial biology. Spatial CITE-seq (spatial co-indexing of transcriptomes and epitopes for multi-omics mapping by NGS), with a wide range of applications in biomedical research has the potential to detect > 200 protein markers in situ along with transcriptome via DBiT-seq technology, using a cocktail of antibody-derived DNA tags (ADTs).
Spatial metabolomics, a field that analyzes the distribution of metabolites within biological samples, has gained significant traction in recent years. Among the various techniques employed for spatial metabolomics, imaging mass spectrometry (IMS) has emerged as a powerful tool. IMS combines the spatial resolution of imaging techniques with the analytical capabilities of mass spectrometry, allowing for the detection and localization of metabolites, lipids, and other small molecules within biological tissues.
Traditional super resolution microscopy techniques limit scientists to observe only a handful of targets at a time. To overcome this limitation, Yale scientists have developed a groundbreaking technique called FLASH-PAINT. This innovative approach enables researchers to visualize a potentially unlimited number of different molecules within a single cell. The core of FLASH-PAINT lies in the novel application of imaging probes. These molecular tools, when combined with highly flexible adapters that rapidly switch between targets, FLASH-PAINT significantly accelerates the imaging process, making it 100 times faster than traditional techniques allowing for comprehensive cellular imaging. Unlike previous methods that required a specific probe for each target, FLASH-PAINT's adapter system offers unparalleled versatility coupled with reduced costs.
Existing spatial multiomics platforms often provide limited information, capturing only a single molecular layer (e.g., proteome, transcriptome) within a tissue. Innovative approaches like IN-DEPTH (IN-situ Detailed Phenotyping To High-resolution transcriptomics) overcomes this limitation. Developed by Harvard scientists, IN-DEPTH integrates spatial proteomics and transcriptomics analysis on the same tissue section, preserving both protein and RNA integrity. This technology is compatible with various commercial platforms and, when combined with k-bandlimited Spectral Graph Cross-Correlation (SGCC) analysis, enables precise single-cell characterization and the capture of cell-type-specific transcriptomes. Other platforms like SMOx (spatial multi-omics data integration pipeline) integrate spatial omics data (Spatial Transcriptomics, Mass Spectrometry Imaging, and snRNA-seq) into a unified framework. This empowers researchers to seamlessly combine diverse data types, unlocking deeper insights into complex biological systems.
The implications of spatial multiomics are far-reaching. By shedding light on the intricate workings of cells, spatial biology's innovative techniques may unlock new insights into the mysteries of life itself.
